
Fuel, blanket and breeding
A fusion reactor uses two isotopes of hydrogen as fuel - deuterium and tritium. Deuterium is present in sea water at a concentration of approximately 115 parts per million. Tritium occurs naturally, although in very small quantities. When fused together, these form a helium nucleus with energy of 3.5MeV and a single neutron with energy 14MeV.
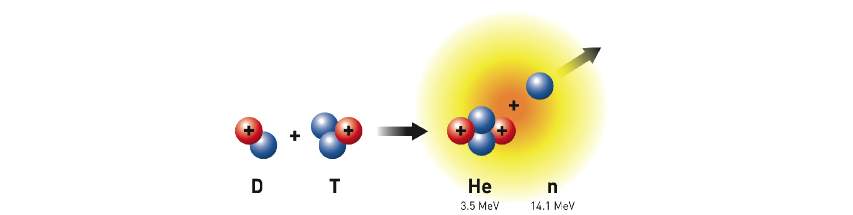
Capturing the energy of the helium nucleus and the neutron is key to retrieving the energy generated from the fusion reaction as heat. This will be performed in a “blanket” surrounding the reactor, covering it as completely as possible to maximise the percentage of neutrons captured.
With tritium being so rare, the blanket performs a second function, “breeding” tritium for recovery and use as new fuel.
For the blanket the ideal material is naturally occurring lithium, commonly used in mobile phone batteries. Lithium occurs naturally in two isotopes, Li6 and Li7. The reactions of these two forms with a neutron are shown below:
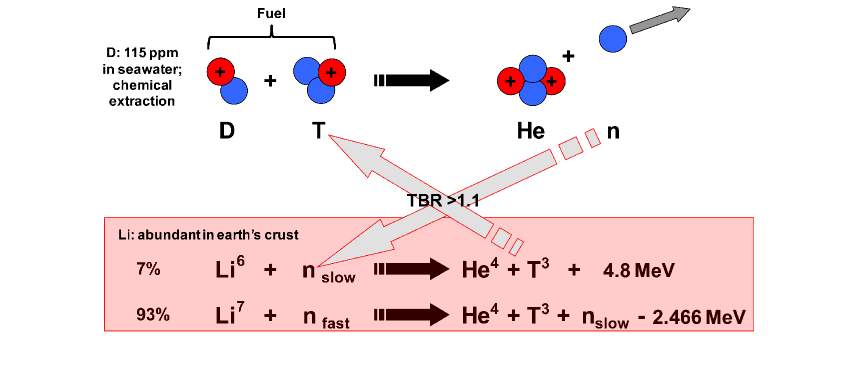
A high energy neutron will react with Li7 producing helium, tritium and a slow neutron, which in turn reacts with Li6, producing helium and tritium, but also giving a bonus in the form of energy. Thus a single neutron can produce additional tritium fuel. As some tritium will be lost in the process and start-up fuel is required for new reactors, varying the isotopic composition of the blanket can be used to determine the Tritium Breeding Ratio (TBR) to produce more or less Tritium as required.
Lithium has a melting point of 180.5°C and a boiling point of 1342°C. This low melting point and high boiling point make liquid lithium ideal as a primary heat energy extraction fluid from the reactor. It may be kept liquid by pumping it through restrictors to induce heat during periods of reactor maintenance. Its high boiling point allows its use to operate reactors at high temperatures, suitable for high temperature high conversion efficiency electrical energy generating plant.
Tritium can be extracted from Lithium by a salt extraction process before refining and recycling as fuel.
First wall
A further challenge in designing a fusion reactor is the wall facing the fusion plasma. Each small pellet fused within the reactor releases a large amount of energy - concentrated into a period measured in micro-seconds. The short timescale of the fusion reaction means that reactor wall components encounter bursts of energy sufficiently intense to damage structures. Even tungsten is melted and its surface damaged at such high energy densities.
Specially structured first wall materials are therefore required. Increased surface area with good conduction reduces the heat load per unit area on the wall to within acceptable margins and materials must also be sufficiently porous to allow helium nuclei, formed in the fusion reaction, to enter the surface before slowly re-emerging as helium gas. Suitably structured porous surfaces permit the helium to migrate.
Materials such as Silicon Carbide and Carbon Nanotubes are being considered and tungsten remains a possibility, if used in a suitably nano-structured surface.
Activated materials
Materials subjected to neutron bombardment become 'activated'. Lower energy neutrons can 'stick' within their structures causing the formation of isotopes which can be radioactive. Through careful selection of materials, the half life of these isotopes can be minimised, allowing recycling of a reactor within 100 years.
